VT Symposium 2025: Dr. Vivek Reddy Shares First-in-Human Results with a Novel Pericardial Access System
Philadelphia, PA – VT Symposium 2025:
At this year’s meeting, Dr. Vivek Y. Reddy presented findings from the Epicardial Access StudY with Rook (EASY-R) – a first-in-human confirmatory trial evaluating the PeriCross™ Epicardial Access Kit (formerly Rook®) from CIRCA Scientific.
Conducted at Na Homolce Hospital (Prague, CZ) under the direction of Prof. Petr Neužil and Dr. Vivek Reddy, EASY-R enrolled 39 patients requiring pericardial access for procedures such as VT ablation, AF ablation, lead extraction, or diagnostic fluid sampling.
Key Findings
- All 39 patients achieved guidewire access to the pericardial space using the PeriCross device, confirmed under fluoroscopy – meeting the study’s primary endpoint
- Average time to access was 3 ± 3 minutes, with over 50 % of cases completed in under 2 minutes – demonstrating quick, repeatable performance
- The incidence of ventricular puncture was 7.7 %, significantly below the 20 % rate historically reported for Tuohy-needle access (p = 0.0058). No surgeries were required, and all events were managed percutaneously.
- Mean fluoroscopy time was 2.0 ± 1.3 minutes, and mean contrast use was 0.7 ± 0.5 mL, minimizing procedural exposure
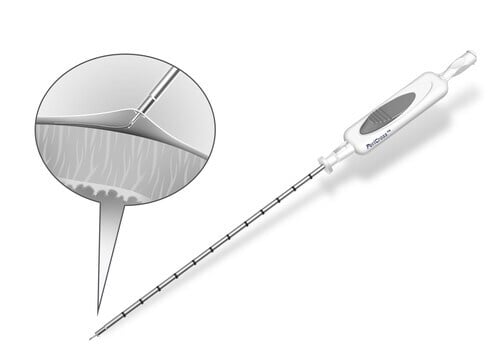
A Stepwise, Purpose-Built Approach
Dr. Reddy emphasized the intuitive design of the PeriCross system – engineered to give physicians control when every movement matters.
-
Tunneler positioned to the pericardial surface.
-
Tines engaged to gently capture and stabilize the pericardium.
-
Pericardium retracted, creating separation from the myocardium.
-
Needle advanced only after capture, enabling deliberate entry.
-
Guidewire deployed into the pericardial space.
The integrated safety-lockout prevents the needle from advancing until the tines are fully deployed, supporting a capture-first, puncture-second sequence that helps reduce risk
Clinical Perspective
According to Dr. Reddy, these results reinforce the potential for a more controlled and confident approach to epicardial access – without changing the familiar subxiphoid workflow. “In this early experience, the device was straightforward to use and provided consistent access in just a few minutes,” he noted during his presentation.
To see the presentation, and more discussoin on the early experiences with PeriCross, visit: www.circascientific.com/easyr
About Circa Scientific:
CIRCA Scientific is dedicated to empowering physicians with innovative technologies that offer unprecedented access and control for complex medical interventions. Guided by a CardioCentric™ approach, we are committed to enhancing procedural success and advancing patient care. For more information on CIRCA Scientific and its portfolio of cardiac solutions, visit www.circascientific.com.
© CIRCA Scientific, Inc., 2025. All rights reserved. CrossWise, CardioCentric, CIRCA Scientific, and the CIRCA Scientific logo are trademarks of CIRCA Scientific, Inc. Patents: www.circascientific.com/patents. CAUTION: US law restricts this device to sale by or on the order of a physician. Before use, consult instructions for use supplied with this device for indications, contraindications, side effects, warnings, and precautions.
SOURCE CIRCA Scientific, Inc.
1 - CIRCA Scientific. Epicardial Access StudY with Rook (EASY-R) – Final Clinical Study Report. M064-097 Rev 00 (2025).

.svg)
.svg)
.svg)

